Snow Play and the Science of Warmth
Field Trips for All of Us: Transformative Adventures for Children and Their Adults
Introduction
Playing in the snow is a blast for kids of all ages. However, feeling cold is not. Staying warm when it gets cold outside is a vital skill. Cold weather, particularly with snow, provides a perfect context for learning the science of heat. The science of heat provides a foundation for knowing how to stay warm, or get warm, when it’s cold outside. This chapter includes many activities and questions for engaging kids in making sense of their experiences of being cold and getting warm as they are sledding, building with snow, or just enjoying the wonders of winter. It also includes a fun and enlivening way of enacting the molecular kinetic theory of heat that helps kids ages 3-133 understand a topic that remains a mystery to most adults.
Activities
Asking Sense-Making Questions: One thing I like to do when I’m out in the snow with kids is to ask them questions about their physical experiences. I usually just ask these questions opportunistically to one or two kids as we’re playing in the snow.
What does snow feel like?
Does it have a smell?
Does it make a sound when it falls?
What color is it?
Does it have a taste?
What happens when we hold snow in our bare hands?
What happens when you squeeze snow and why?
What happens to snow when you hold it in a gloved and ungloved hand?
How does your hand feel when you hold snow in it with your gloves off compared to when you hold it with your gloves on?
How many types of snow can you find?
How do you feel in the wind? Does it warm you up or cool you down?
What happens to the snow after you sled over it multiple times?
Why do we seem to slide on snow when it’s a bunch of solid spikey bits? (hint: sleds don’t slide on snow, they slide on a thin layer of snow that melts (i.e. water) from the friction and pressure from you sledding over it).
Why Does your hand get cold and the snow melts when you hold it in your hands?
Why do we slide downhill instead of up?
Why is it harder walking uphill than downhill?
Why do we breathe harder when we’re walking uphill?
Why do we get warmer when we’re walking uphill?
How do icicles form?
Why can we see our out-breaths?
Where does the snow come from?
Where does snow go when it leaves?
Do clothes make us warm? Do certain fabrics keep us warmer than others?
How can you warm up if you feel cold?
How can you cool down if you feel hot?
How do we warm up and cool down our houses?
The above are just a sampling of questions at different levels that we can ask children based on their embodied experiences. And you don’t have to know the answers to the questions you ask! You can research them together later, or not. Open-ended questions are a good thing! They encourage curiosity, while supporting the kids’ critical thinking and confidence in their ideas and opinions. Note, that not all questions are created equal. I tried to order the above questions from those that stick close to the physical experience (e.g., How does snow feel in your hand?) to those that are more conceptual (e.g., Why does your hand get cold and the snow melt when you hold it in your hand?). Younger children probably won’t be able to do much with the more conceptual questions but asking them those questions certainly won’t hurt them! Older children will be able to contemplate the more conceptual questions and will be way more interested in such contemplation because those questions grew out of their direct experience and built upon more experiential questions.
Observations and Demonstrations of Heat and Temperature
Demonstrating Heat Flow via Conduction: While enjoying some hot tea or hot chocolate you can demonstrate heat flow by having the kids feel the temperature of the liquid or the hot mug and then of snow. Perhaps even dropping some snow in the hot liquid If you have an infrared thermometer, you can get the temperature of the liquid and ice before putting them together and then compare the temp of the tea after you’ve put the ice in. Ask them what happens. They’ll probably note that the snow melted and the liquid got cooler. Now you can talk about how the liquid was hot and the snow was cold and after you put them together the snow got warmer and melted and the liquid got cooler. It’s as if the heat flowed from the liquid to the ice. Now ask the kids to hold a little snow in their bare hands. What happens? Why? After some discussion I, often and repeatedly, point out that when heat flows from a hot material to a cold material through touch, scientists call it conduction!
Demonstrating Insulation: Insulation plays a major role in staying warm while being outside in the cold. Insulation is anything that interferes with the flow of heat from warmer to cooler things. Jackets and gloves are insulation that stops heat from flowing from our warm bodies to the cold air and the snow around us. Some kids think jackets, etc. are warming. However,it’s important to recognize that we are bio-heaters that generate warmth! The insulated fabric just keeps the heat inside and close to our body. One way to demonstrate this is to cover some snow with a spare jacket and see if it melts the snow. Another way of talking about insulation is noticing that materials that are good insulators are also bad conductors of heat. Water is a great heat insulator, which means it’s a bad conductor. It’s such a bad conductor that it turns good insulation like gloves and jackets into good conductors of heat. That’s why it’s important to change from wet to dry clothing in cold weather!
Demonstrating Convection: When it’s windy out, I like to ask kids if they feel warmer in or out of the wind. When the air is still, the air near us that has been warmed by the conduction of heat from our bodies stays close to us. When it's windy, the wind blows that warm air away from our bodies and replaces it with cooler air at the speed of the wind! The movement of warm materials from one place to another is called convection. Adults can also demonstrate convection by holding a smoking stick and asking the kids to notice where the smoke goes. The smoke is made of hot air with partially condensed water vapor and partially combusted hydrocarbons, which is what makes it visible to our eyes. You can also demonstrate convection with a lighter. Have older kids explore the temperature of the air to the side and above the lighter. It’s warmer above then it is to the side of the lighter. This is because the hot air (and other fluids) rise as hot air is less dense than cool air. Cool air moves continuously to fill in the space left by the warm air rising up. These actions create convection currents. These currents are partially responsible for wind and its patterns.
Demonstrating Thermal Radiation: You’ve already met two of the ways that heat flows: induction and convection. The third and final method for heat transfer is thermal radiation. Radiation is often the most confusing form, in part because when people hear the word, they think of ionizing radiation. This is a form of radiation that causes radiation sickness. However, thermal radiation is the transfer of heat via the electromagnetic waves. Thermal radiation is why we are warmer when we stand in the sun; shade blocks the thermal radiation coming from the sun. There are at least three easy ways to demonstrate the impact of solar radiation. The first is to ask kids to move from direct sunlight to shade and ask them to describe their experience. Another is to have them face a fire and describe which part of them warms up the fastest. Personally, I think the most fun way is to use a hand lens to focus the sun’s thermal energy onto a spot of snow and melt the snow!
Summarizing Practical Ways to Warm Up or Cool Down: Being in the sun, keeping dry, protecting ourselves from wind with clothing or shelter, being well insulated, and being active are all ways to get and keep ourselves warm. We are warmed by the sun’s thermal radiation when we’re in the sun. Staying dry prevents us from losing heat via conduction as water is such a good thermal conductor. Protecting ourselves from the wind keeps us from losing heat through convection. Being well insulated prevents us from losing heat through conduction, convection, and radiation. And being active leads to bio-heaters, like us, generating more heat by slowly burning sugars in our cells. For all of the same reasons, being in the shade, wetting our skin, being still, fanning ourselves, and removing layers of clothing are all good ways to cool our bodies down.
Use Infrared or Other Thermometers to Explore the Temperature of Various Surfaces: If you have one or more infrared or other robust thermometers, I’d recommend letting kids that can use them, do so and check out the temperatures of things as you wander the forest.
Using Theater to Model State Transitions
Most of the following activities are most suitable for older kids. As always, we believe there is no harm in trying them with any kids old enough to respond to verbal requests as long as you, the adult, don’t worry about how much they are getting and trust that they will always get something from it.
Noting that Water Comes in Three Forms or States: A snowy day is a wonderful time for children to observe water in a variety of states and for us to help them make sense of their observations. When we breathe out, the water vapor (water in the form of a gas) in our breath cools and becomes a mist of tiny water droplets suspended in air. When we hold a pinch of snow between our bare fingers, solid water melts and becomes liquid water. When we burn wet wood, water evaporates from the wood and condenses into a mist of steam that is mixed together with partially combusted hydrocarbons to form smoke above our fire. After some direct experience, I’ll ask kids if snow is a solid, liquid, or gas. Then I’ll ask about water and farts. For kids who can match each material with the correct state of matter, I’ll ask them how they know snow is a solid, water is a liquid, and a fart is a gas. Again, leading with curiosity and open-ended questions to encourage their ideas and hear what is relevant to them and their experiences.
Making Sense of Energy: When I engage kids in making sense of energy I start off by asking them what they think of when they hear the word energy. Everytime I can remember, kids associate lots of energy with lots of motion! I typically end this conversation by pointing out that scientists call the energy of motion, kinetic energy.
Modeling Kinetic Energy: To enrich the connection between this word and kids’ intuitive ideas about energy, I’ll often ask them to show me how they act when they have low and then high kinetic energy. If they’re having fun enacting their energy levels I’ll call out, show me high energy, now medium, now low, and so on. You can continue to build off this by adding in questions such as, “show me your level of kinetic energy while you are cooking breakfast? What about taking a bath? Sleeping? Playing tree tag?”.
Modeling Molecular Kinetic Energy in a Solid: After they’ve modeled kinetic energy, usually by running around at different speeds, I’ll ask kids to stand close together, without moving from their spot, show me low, medium, and high energy. Then I tell them that they just acted out what happens when a rock gets hot! Rocks are made of tiny little pieces called molecules, much smaller than a grain of sand. So small we can’t see them in visible light, but we can see them with support from tools like X-rays or an atomic force microscope. When a rock gets hot from being close to the fire, those tiny molecules start moving with more and more energy, but in solids, even hot ones, the molecules stick very close together. The reason we can get burned by touching a hot rock is that the fast moving molecules in the rock get the molecules in our skin moving so fast that our skin cells brake apart.
Modeling Molecular Kinetic Energy in a Liquid: Next, I’ll ask kids to stand close together, but now quite as close as when they were a solid, and move quickly past each other (I often join in to model the kind of movement). Then I tell them that they just acted out how molecules move in a liquid! The way molecules in water slide around lets them get out of the way when we drop an ice cube into a glass of water.
Modeling Molecular Kinetic Energy in a Gas: Finally, I’ll ask kids to stand far apart and run randomly all over the place. Then I tell them that they just acted out being a gas! The way gas molecules bounce around all over the place is why, when someone farts, they are the first to smell but soon the smell spreads all over the place!
Modeling Melting and Freezing: Now the fun really begins! First, I ask them what they think of when they hear the words melting and freezing. Sometimes I’ll focus on water and ask what happens when liquid water freezes and when ice(solid water) melts. I usually summarize or clarify their answers when they’re done: freezing is when a liquid becomes a solid and melting is when a solid becomes a liquid. Afterwards,I’ll ask them to start off standing close together like molecules in a solid and to act out whatever I say. eat up, then melt, then freeze, and layer it by adding different states of water they can see or touch such as, acting like water as an icicle, steam, or in a mug!
Modeling Evaporating and Condensing: After asking kids to tell me what they think of when they hear the words melting and freezing, I sometimes demonstrate them before asking the kids to model them. You can demonstrate both by building water over a fire and holding a piece of cold metal in the steam above the boiling water. The steam will further condense on the metal and after a while you can take the water off the fire and show them that some of the water has evaporated. Then I’ll ask them to start off standing close together like molecules in a solid and to act out what I say and have them move from state to state for as long as they are having fun.
Some Nature Science
The Molecular Kinetic Theory of Heat: The main idea of this theory is that heat is a product of molecules in motion. Within the molecular theory of heat, Heat is defined as the movement of thermal energy between materials. Thermal conduction is the flow of heat between materials in contact with each other. Conduction occurs when the fast moving (high energy) particles in warmer matter collide with the slower moving particles (low energy) in cooler matter. This slows down the particles in the warmer matter (reduces their energy) cooling that matter down. It also speeds up the particles in the cooler matter (raises their energy), warming that matter up. I sometimes explain this to older kids by having them imagine grains of sand on a drum head. Convection happens when hotter fluids (liquids and solids) rise, creating convections currents. Radiation happens when objects above absolute 0 release electromagnetic waves in frequencies proportional to their temperature (IR radiation corresponds to the motion of atoms in objects around room temperature). All warm objects emit thermal radiation in the form of electromagnetic waves (we call visible electromagnetic waves, light). When these waves strike a particle the particle’s motion is changed, increasing their energy of motion and thus their temperature. Infrared waves are best at increasing the molecular kinetic energy of particles at around human body temperature and are the wavelength that is mostly emitted by materials at around the same temperature about 36.5–37.5 °C or 97-98.6°F, though babies and kids can be a bit higher. Unlike conduction and convection, heat flow via electromagnetic radiation does not depend on matter.
Temperature is a measure of the average kinetic energy (energy of movement) of the particles in a material.The kinetic energy of any system is the motion of the objects in that system.
KE=1/2MV2
While I don’t have this formula memorized, I do think it is useful to remember that the average kinetic energy of the molecules in an object and thus its temperature is proportional to both the mass and velocity of the particles involved and velocity is “more important” than mass.
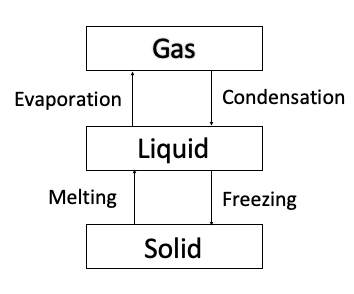
The Molecular Theory of Heat Applied to State Transitions: Almost all matter can exist in one of four states: solid, liquid, gas, and plasma. We’re going to focus on the first three here. As illustrated below, the state transitions between these three states are melting, evaporating, condensing, and freezing.
For example, water can exist as a solid that we call ice. It can exist as a liquid we call water. Lastly, it can exist as a gas, which we call water vapor. The main property of solids is that they maintain their shape. For example, ice cubes and snowballs maintain their shape (unless they melt). Liquids maintain their volume (i.e., how much space they take up) but can change shape and through gravity, take the shape of their container. While, gasses have no fixed shape or volume and are often invisible.
Molecules in solids are similar to running in place. They keep their positions relative to each other but move faster or slower in place depending on the temperature of the material they are part of. Within solids the molecular kinetic energy is not enough to overcome intermolecular forces, so the molecules in the substance remain stuck together. However, molecules in liquids slide past each other. Their molecular kinetic energy is high enough for them to move away from each other but not high enough to break their collective attraction, so they stay together as a group. On the other hand, molecules in a gas have enough energy to totally overcome intermolecular forces, so they just bounce around all over the place! Lastly, plasma is matter with so much energy that many atoms are separated into ionized nuclei and “free” electrons. For example, the light from a fluorescent bulb is raising the energy of gasses in a tube so those gasses become plasma.
What is a Theory? Some people believe that a theory is somehow less than a fact and therefore less true. A fact is something that has been verified by experience. A hypothesis is less than a fact. an It is an educated guess that can be tested. Facts and hypotheses are small things. Theories are grand. Theories explain and are supported by a large number of facts and can be used to make predictions. Molecular kinetic theory explains and predicts so much about heat and states of matter just as the theory of evolution explains and predicts so much about life.
Educational Ideas
In factory-like schools, teachers use lectures to transmit information to students. In totally child-directed learning environments, adults keep in the background and wait for requests from children before offering assistance. In ecological learning environments, we synthesize these two patterns. We give kids plenty of time for free play and self-directed activity. And, when the time is right, we interject questions, demonstrations, and sense-making conversations. We also recognize that learning often happens gradually, over time and through repeated encounters with experiences, concepts, and language. This frees us from worrying about sharing ideas that kids might not yet understand and helps us relax about kids having half formed ideas.
Wrap Up
The ability to maintain our core temperature is essential to our survival. There are many practical things we can do to warm up and cool down our bodies. Understanding the science of heat can help us make decisions that will allow us to maintain our core temperature within a healthy range. Playing in the snow provides a great context and lots of motivation for making sense of the science of heat. Asking kids questions about their experiences with cold, guiding their observations, doing outdoor demonstrations, and using theater are all useful and fun ways to help kids come to understand heat and state transitions. For a great video full of activities and tips for staying comfortable outside in the snow see Tips for Outdoor Play in the Winter, by my friend, Krista Freel!




Hi. This sounds amazing.
I would love to connect with the field trips for my 2 kiddos.
Hey Jennifer, What do you mean by saying, "I would love to connect with the field trips for my 2 kiddos." Are you looking for an in -person field trip or saying you plan on doing the activities in post with your kids, or...?