Building Fires and Understanding How the Sun is the Source of all of the Fires of Life
Explorations in Ecology: Playful Science-Rich Outdoor Activities for Children and Their Adults
Note: The first 30+ posts in this blog are source material for an in-process book, Explorations in Ecology: Playful Science-Rich Outdoor Activities for Children and Their Adults. More recent posts are plans and reflections from Exploration in Ecology meetups I host for children and my current musings about ecological education. To receive new posts, become a free subscriber. To support our work, buy the book when it’s released in October of 2026. Here is the preface. The first field trip is Taking Education Outside.
The Sun
We now send greetings and thanks to our eldest Brother, the Sun. Each day without fail he travels the sky from east to west, bringing the light of a new day. He is the source of all the fires of life. With one mind, we send greetings and thanks to our Brother, the Sun.
Now our minds are one.
From the Haudenosaunee Thanksgiving Address, National Museum of the American Indian
Introduction
Building and circling around fires is a uniquely human activity (there aren’t many) that stirs something ancient in us all! Learning to control fire was a tremendous leap for humans, enabling us to keep warm, protect ourselves from predators, make water safe for drinking, and cook food. Starting and building fires remain essential skills. Even in my on-grid home, we have had times when we had no electricity, no propane, and could not be reached by propane trucks. We would have been in trouble without a fireplace and fire-starting skills.
Despite the importance of energy to the human condition, very few adults understand what it is or how the Sun is the source of the fires of life. And, notwithstanding the importance of fire skills, fewer and fewer people practice them. In this post, we suggest activities and conversations to support your group in learning these critical ancestral skills and understanding the modern science of how the Sun is the source of the fires of life.
Activities
Rather than planning a session to focus on energy, we like to use the following activities opportunistically as we encounter different environmental conditions in the forest and engage in activities that relate to how the Sun is the source of all of the fires of life. Because there is a sequential flow to these topics, consider doing them in order. They follow the Sun’s energy from shining on us and plants, to plants using it to synthesize fuel, to us burning fuel outside and inside our bodies.
Listening to Children’s Ideas about Energy
Assessing the quality of the final product is an important job in any factory or factory-like school. As a nature guide and educator, I’ve always been very interested in learning about folks' prior experiences and ways of learning, being, and thinking about the world. Asking kids what they know about something can be an intimidating question.
Ask the kids in your care what pops into their minds when they hear the word energy. If you have a way of recording their answers (using your phone or a notepad), do that. At first, avoid evaluating, or elaborating on their answers.
Many kids and adults associate the idea of energy with their activity saying things like, sometimes I have lots of energy and sometimes I don’t. That’s a good place to start from as energy is indeed associated with activity.
Sun, Shade, and Shadow Tag
Given that the Sun is the ultimate source of almost all energy on Earth, it’s not surprising that one direct way we experience energy is when sunlight hits our bodies. On sunny days, suggest to kids that they stand in the sun for a minute and then in the shade and describe how their exposed skin felt in both situations.
Ask them if they feel different when they're standing in a sunny area versus a shaded area.
Ask them why they think they feel warmer in the Sun.
A child in one of my classes invented a game that is also a useful context for this discussion. He called it “shadow tag.” The basic idea is that kids are on base when their shadow is hidden behind another shadow.
Depending on the kinds of answers I get, I tell some version of the following science story incorporating their answers where they fit.
Light beams are amazing. They can travel through outer space. When light beams that have traveled millions of miles through the vast emptiness of space hit us or anything else, the light energy changes into heat energy. That’s why we feel warmer in the Sun than in the shade.
Where’s the Snow
Another way we experience light energy becoming heat energy is when we observe where snow has melted and where it hasn’t in a given area. I like to point out differentially melting snow to kids and ask them for their explanations. After listening, I engage them in making sense of their observations and usually share my thoughts at the end.
Those same light beams that turn into heat energy and warm us when they hit us also turn snow from solid water to liquid water.
Sharing Life-Giving Breaths with Plants and Receiving their Gift of Sugar
In addition to warming the Earth and all of its inhabitants, some communities, such as the Haudenosaunee people know that the Sun, “ is the source of all the fires of life.” When I’m wandering the forest with kids, we find edible berries (i.e., wild strawberries, blackberries, currants, manzanita berries, coffee berries) or flowers with edible nectar (e.g., honeysuckle), I like to do the following (Of course, don’t let kids eat anything unless you are certain of its edibility):
Share with your kids that some communities, such as the Haudenosaunee people know that the Sun, “is the source of all the fires of life.”
Ask them if they have any ideas about what that means.
Ask them to find a spot where they can stick their faces up close to where the berries are growing (you might have to caution them to be careful as many berry bushes are thorny) and breathe into the bush for a few moments.
Suggest they taste a berry (I check about allergies when kids enroll in one of my sessions).
As they’re eating, ask them if anyone knows why the berries taste sweet. Often, kids will know that berries have sugar in them. If not, I’ll share that information with them.
Next, ask them if any of them know anything about how plants make sugar. I also often offer up some more guiding questions: what do plants do with sunlight? What do plants make from our outbreaths, or What do plants breathe in and out? Sometimes I’ll mention the word photosynthesis to see if that sparks anything for them but more often than not, that word prompts a regurgitation of their attempted memorization of some not fully understood concepts.
As always, I use their answers to form a customized version of the following story. I’ve put more “advanced ideas” in square brackets to indicate that they are add-ons if the group seems like they’ll gain something from hearing about them or at least won’t be confused by the added details.
Plants have the amazing ability to use our outbreaths [the carbon dioxide; C02] to make sugar that we can benefit from. Scientists call the process of making sugar from carbon dioxide with energy from the sun, photosynthesis. We animals evolved by getting our energy from eating plants or other animals or fungi that digest plants and burning the fuel we get from those other living things. So, when you eat berries, nectar, or maple syrup, you're eating and tasting sugar the plant made to store its energy.
I also like to share this quote from Braiding Sweetgrass, my favorite book of all time, written by Robin Wall Kimmerer, a Potawatomi botanist, author, and professor, says, “ The syrup we pour over our pancakes on a winter morning is summer sunshine flowing in golden streams to pool on our plates.” We highly recommend this book, as well as the Young Adult version!
If kids seem comfortable with these ideas, I sometimes add a little more detail.
Plants contain chloroplasts. Even though chloroplasts are tiny, in many ways they are one of the most powerful things on Earth. These tiny little green chloroplasts capture the energy that the Sun beams out into the universe and use that energy to make sugar. Sugar is like a battery for many living things. It stores the Sun’s energy and plants use that energy to fuel their growth, reproduction, and other things that plants do. They also use sugars as building blocks to make bigger combined building blocks called carbohydrates. Roots, stems, leaves, flowers, fruits, seeds, and sap all contain carbohydrates.
Roasting Marshmallows and Burning sugar
One of the most direct ways to help kids connect the idea that plants make sugar to the idea that sugar is a storehouse for energy is to burn some sugar. And a fun way to do that is to roast some marshmallows! This example is powerful because sugar (the main ingredient in marshmallows) burns easily when lit with a lighter, over campfires, and in the “little fires” in our cells!
If you’re doing campfires at this time:
Demonstrate what happens when you stick a marshmallow into a flame by just sticking one on a thin green branch and holding it in the fire.
If using a fire isn’t convenient at this time:
Demonstrate what happens when you hold a strong lighter under a marshmallow.
In either case:
Ask your kids why they think the marshmallow burned so easily. Does everything burn that easily? Why or why not?
Again, depending on the kinds of answers I get I tell some version of a story weaving in their answers.
I think sugar burns so easily because it stores a lot of the Sun's energy in a way that is easy to release as heat and light!
Discussing the Power of Fire
Before delving deeper into how energy from the sun fuels life’s fires, which involves starting some external fires, it’s essential that kids develop a healthy respect for the power of fire. The ability to harness the power of fire was one of the most important discoveries of human prehistory. Like almost all human technology, fire’s utility as a source of heat and light is more than balanced by its potential to cause harm. I like to start discussions like these with mostly open-ended questions connected to activities that are meaningful to the kids. This is a discussion I like to have as we’re setting up to work with fire for the first time and I repeat some of the questions every time we work with fire.
What is fire good for? What does fire do for us? How can we use fire?
Is fire also dangerous? If so, Why?
What can we do to make our work with fire safer?
Does this look like a good spot for a fire? Why or why not?
Fire requires a small input of energy to get started. Once started, fires release way more energy than they need to keep going. This excess of energy increases the size of the fire. This is why fires, in the presence of abundant fuel, can easily get out of control. For this reason, fire safety precautions are vital. If the kids don’t come up with any of the following, I share the following precautions with the kids:
We will always follow local fire safety regulations,
We will only start fires where the surrounding area (including above the fire) is clear of combustible materials (things that burn),
We will only have fires within a fire circle we create with rocks (or some other safe container) to help contain the fire, and
We will never take anything out of a fire once it's been in (except for carefully removing food).
Building a One-Match Fire
Once you’ve selected and prepared a fire-safe area you’re ready to set your kids up to practice starting a fire with a single match. I like to have kids do this in groups of two to four. I like to give them a lot of freedom. For their first try, I give them the following instructions:
Prepare your spot and call me over to check it out. Then collect your materials, prepare your materials to be lit, and any materials you want to have on hand, and call me over. If it looks like you’re ready, I’ll give you one and only one match, watch your initial attempt, and give you some feedback.
Nurturing fires, like many basic nature skills, requires patience. Beginning firemakers often fall victim to the “more is better” fallacy and add fuel so fast that there is not enough energy being released to keep the fire going. Like learning anything complicated, learning about fire building is an iterative cyclical process: try something, observe what happens, reflect, discuss, and try again. There are many lessons to be learned including using only the driest material you can find (often dead tree parts still on the tree) and starting with the smallest materials you can find (i.e., tinder) and building up to the next size up (i.e., kindling), and so on from there. I also like to introduce kids to using pine sap and pitch as fire accelerants.
Once kids have their fires going:
Ask them if it was as easy starting their fires as it was to light a marshmallow. Chances are, they’ll say no.
Ask them why they think it is harder to get a stick to burn than it is to get a marshmallow to burn.
The story I tell kids goes something like this.
One difference between burning marshmallows and wood is that the sugar in marshmallows is very close to being ready to burn. Imagine sugar being like an easy-to-burn Lego. The sugar in wood has mostly been joined together with other sugars a lot like putting a bunch of Legos together. [These joined sugars are called complex carbohydrates]. Before complex carbohydrates can burn they have to be broken apart. When we burn wood we have to supply a lot of heat energy to break the sugars apart before they can burn.
Starting a No Match Fire with a Hand Lens
There is plenty of information about starting fires without matches available in survival guides and on the Internet. We’re going to briefly introduce three methods here and suggest you get good at them before trying to help anyone else with them. The three methods are: (a) using a hand lens, (b) using a ferro rod, and (c) using a bow drill.
If you have a hand lens, find an object you want to magnify. Hold the lens between the object and your eyes at a 90° angle to your line of sight. Now move the lens back and forth between the object and your eyes until the object is in focus. Once you can do this, help children do what you did, one at a time. Exactly how lenses work is a story for another time. For now, know that when a lens is used like this, it spreads out light rays reflected by small objects and projects those rays onto your retina (i.e., the “screen” at the back of your eye). That makes the object appear larger.
Now, find a spot in direct sunlight where you can clear a circle ten feet wide of any combustible material. Place a dry leaf in the center of your circle. Hold the lens between the leaf and the sun at a 90° angle to your line from the sun to the leaf. Now move the lens closer and farther away from the object projecting as small of a circle of light onto the leaf as possible. If things are lined up right, you'll first see some smoke, then fire.
Starting a Fire with a Ferro Rod
Ferro rods are available at outdoor stores and on the Internet. They are a modern improvement of starting fires by striking iron against flint to generate a spark and aiming that spark at your tinder. Ferro rod is an abbreviation of Ferrocerium rod. Ferrocerium is an alloy that when struck by a hard object, generates a high-temperature spark that can be used to start a fire. Again, acquire one if you can, get good at using it, and then model and guide its use by kids. You’ll need very fine tinder to catch a spark from your ferro rod.
As we describe in more detail in the Science section of this post, it takes a lot of energy to get any solid to the point where it starts to burn and release stored energy. As the kids begin to work with Ferro Rods I tell them this science story.
The sparks from your ferro rod carry just a twinkle of energy. For them to do the work of breaking up the strings of sugar [complex carbohydrates] in the plant fibers, and then breaking up the sugar to release flammable gasses [mostly hydrogen and methane], and then igniting those gasses, the plant fibers must be very fine. Wispy, feathery, filaments of plant fiber do the best job of igniting when touched by a spark or ember because they present the maximum surface area where the spark can split a string of sugars into hydrogen and methane and ignite those combustible gasses in the presence of an oxygen molecule or two.
Making a Bow Drill Set
Starting a fire by rubbing sticks together is an important basic skill. Ricardo Sierra uses the phrase, “the skills behind the skills” to talk about all of the skills that are prerequisites to a given expertise. In the case of starting a friction fire, these skills include finding things in the forest, basic carving skills, a feel for how to balance a fire’s need for oxygen and fuel with the amount of energy the fire is outputting at any given moment, and an understanding of, and feel for really dry combustible wood. Bow drills are the standard for friction fires being somewhat less physically demanding than hand drills and less complicated to construct than a flywheel drill.
Bow drills include four basic components: bow, fireboard, spindle, and spindle holder (not visible in the picture). Again, there’s a lot of information about using and making bow drills available in survival guides and on the Internet. I usually start by demoing using a bow drill and then giving kids guided practice time with my set. Then, I challenge them to find and craft the components one at a time with feedback from me. I suggest they start with a spindle: the straight stick that is used to drill into the fireboard.
The Fires of Life, Walking Up Hill, and Melting Snow
While walking uphill kids will often notice that they are breathing harder and warming up. If they do not notice this themselves, I sometimes point it out.
Are you breathing harder and if so, why?
I notice you're unzipping your coat, are you getting warmer? Why do you think that might be happening?
When we observe snow that has melted away from the base of a tree, kids sometimes point this out. If they don’t, sometimes I do.
Do you see how the snow has melted away near the base of that tree? Why do you think that happened?
After they’ve talked about their answers to these questions, I tell them some version of the following science story.
So far we’ve experienced light energy turning into heat energy by playing around with shadows. Then we breathed into plants and talked about how plants use the energy of the sun to turn [the CO2 from] our outbreaths into sugars which store the sun’s energy to be released and used later. We’ve also used fire to experience releasing the plant’s stored up energy from the sun as light and heat energy. But what about those little fires of life burning inside of us? We have very slow burning low-temperature fires burning inside of us [in every cell in our bodies]. Like fires outside of our bodies, the fires of life use oxygen to burn sugars releasing stored energy. Walking uphill uses a lot of energy so while we’re walking uphill our life fires burn more to give us the energy to make it up the hill. We breathe harder to supply our life fires with oxygen and we heat up because our fires are burning faster.
Science
To help you keep one step ahead of your kids, for now, we provide the following more complete science story for you. Feel free to give it to older students to read if you think it might be helpful and not overwhelming.
Energy is What Makes Stuff Happen. When I talk with kids about matter and energy, I say that everything is made of matter and everything that happens happens because of energy. With older kids, I mention that matter is associated with nouns and energy with verbs. The basic story with energy is that it cannot be created nor destroyed, only transformed from one form to another. The two main forms of energy are kinetic and potential. Kinetic energy is the energy of motion or more generally stuff happening. Potential energy is stored energy that has the potential to make stuff happen. For example, fire transforms potential chemical energy stored in wood into the molecular kinetic energy of heat (heat is the energy of molecules in motion, which is a story for another post). Calories are a common unit of measurement for energy. When we say that there are a few hundred Calories in a candy bar, we’re saying that the molecules in the candy bar have the potential to release a few hundred Calories of energy.
Using the energy of the Sun to synthesize sugars inside of chloroplasts is called photosynthesis. Plants and other photosynthesizing organisms like cyanobacteria, photosynthesize sugar from carbon dioxide and water using the energy of the sun. The summary equation for this chemical reaction is:
Inside chloroplasts, the light energy is transformed into potential chemical energy stored in the arrangement of atoms in the sugar molecule. In addition to energy storage in sugars, plants use sugar molecules to build larger cellulose and lignin macro (large) molecules. These molecules form the structural framework of plants. This is what it means when I tell children that plants build themselves from our outbreathes.
Burning sugar at a low temperature inside of mitochondria is called respiration. Most life on Earth uses oxygen to release the energy stored in food. Processes and organisms that use oxygen to release the energy stored in food are called aerobic. The summary equation for aerobic respiration is:
Sugar burning in the presence of oxygen releases the potential energy stored in the arrangement of atoms in the sugar molecule with water and carbon dioxide as byproducts.
Anaerobic organisms, including anaerobic bacteria, work and play in low-oxygen environments, often in water-saturated environments. Some aerobic organisms, including humans, engage in anaerobic metabolism under special circumstances like those encountered during anaerobic exercise.
Autotrophs and Heterotrophs both respire to release the energy stored in sugars. Many living beings use sugar for energy storage. Green beings make sugar from carbon dioxide and water. Herbivores and omnivores get their sugar from eating plants. Beings that synthesize (or other organic food molecules) are also called autotrophs (literally self-feeders), while those of us who get our energy molecules from other beings are called heterotrophs (other feeders).
Fire, unlike photosynthesis and respiration, is a purely physical phenomenon. Fire presents a fascinating area of study from many points of view including chemistry, physics, biology, history, anthropology, archaeology, literature, art, permaculture, and survival. Here, we take a look at some of the physics and chemistry of fire. In particular, we’ll answer two burning questions: Why do we need to build fires from small to large? And, why does it make sense to say that wood doesn’t burn?
There are three main forms of energy and at least three processes involved in getting fire from plant matter. The three forms of energy are potential chemical energy, light (AKA, electromagnetic radiation), and heat (AKA, thermal energy). The three processes are:
Evaporation of water: a physical change that requires an input of thermal energy (i.e., is endothermic)
Pyrolysis of complex carbohydrates: a chemical change that requires an input of thermal energy (i.e., is endothermic)
The combustion of small flammable gaseous hydrocarbons (e.g., methane) and hydrogen in the presence of oxygen: a chemical change that releases thermal energy (i.e., is exothermic)
Starting a fire requires supplying a small amount of thermal energy in the form of a spark or match or lighter flame to tinder, usually in the form of a very dry complex carbohydrate with a lot of surface area (e.g., the cellulose in paper or lint). That thermal energy is enough to break some complex carbohydrates into flammable gasses through pyrolysis and to combust those gasses.
The thermal energy released from the combustion of the flammable gasses released from the tinder is enough to break some complex carbohydrates in kindling (e.g., small, dry sticks) into flammable gasses through pyrolysis and to combust those gasses. The thermal energy released from the combustion of the flammable gasses released from the kindling is enough to break some complex carbohydrates in full-sized pieces of firewood into flammable gasses through pyrolysis and to combust those gasses. At this point, the fire just requires the addition of firewood to replace that which has been reduced to ash (i.e., partially combusted hydrocarbons). Under most conditions (except when the wood is wet, including green), combustion releases more heat than evaporation and pyrolysis take so we get to be warmed by the fire.
The balancing act to building a fire is to add new materials, and/or build a structure such that new materials come in contact with flame slowly! This slow progression from smaller to larger materials makes sure that the evaporation of water and pyrolysis of carbohydrates from the new material does not require more thermal energy than is currently available from the combustion of the flammable gasses. A small flame gets small materials going. A larger flame gets larger materials going… This is a great example of a positive feedback loop!
Understanding Feedback Loops: Under the linear causality of mechanistic logic, A causes B. In living and cybernetic systems, A causes B causes A causes B, and so on. The two main forms of circular causality are positive and negative feedback loops. The feedback caused by a mic and an amplifier facing each other, painful as it is, is an example of a positive feedback loop. The mike picks up a sound (A). The amplifier makes it louder (B). The mic picks up the amplified sound (A). The amplifier makes it louder (B). This is called a positive feedback loop because the feedback increases the intensity of the original action. While positive feedback loops tend to lead to run-away systems, negative feedback loops tend to keep systems stable. For example, the furnace heats the house (A). The thermostat turns the furnace off when the temperature reaches 68° F (B).
The dominant culture's abuse of energy from fossil fuels has jerked our global ecosystem out of homeostasis. The dominant culture's abuse of energy from fossil fuels has jerked our global ecosystem out of homeostasis. This has led to an increase in extreme weather events which in turn contributes to failing technological infrastructure leading to power outages and water contamination, among other breakdowns. As such breakdowns become increasingly frequent, fire skills become increasingly important for modern humans. An average adult citizen of the USA burns somewhere around 2,300 Calories a day to perform our life functions. According to Nate Haggens, the average adult citizen of the USA uses about 100 times that to heat our houses, power our devices, and otherwise support the lifestyle to which we have become accustomed. This staggering overuse of energy is at least partially responsible for the global climate change and eco- and social-system collapse we are now experiencing. Understanding how energy works also is key to understanding why we must change how we use energy to survive as a species in the post fossil fuel age that is rapidly approaching.
Education
I am a fan of synthesis. The concept of Taiji, which preceded and is incorporated into Taoist thinking, posits that all being results from the integration of the opposites, yin and yang. Three integrations that are relevant to this post are: (1) synthesizing the learning of traditional ecological knowledge and modern science, (2) synergistically melding teacher-centered and child-centered educational methods, and (3) integrating play and academic content.
Synthesizing the Learning of Traditional Ecological Knowledge and Modern Science: I’ve heard people ask, “Should I be supporting my children learning what they’ll need to know to get into college and survive in today's high-tech world, or should I be helping them learn the skills of their ancestors: growing food, staying warm, building shelters.” My answer is, yes. The activities in this field trip, like those in many of the field trips in this series, include both land-based ancestral skills and discussing the natural world in terms of modern scientific ideas. There is no either-or here. Knowing the science behind land-based skills helps us generalize those skills and understand why we do things a certain way. For example, knowing that evaporating water from wood requires energy helps us understand our search for kindling that feels dry and snaps when we try to bend it. Similarly, people learn best when what we’re learning is meaningful to us. Staying warm and providing for our basic needs is about as meaningful as it gets. When I first encountered chemistry, I thought it was a fun puzzle. My high school chemistry teacher sucked all the fun out of chemistry by pouring out an overwhelming onslaught of formulas and facts without any connection to our everyday reality. The kids I work and play with now love the chemistry we learn as we’re building fires and growing food! Not only do they love learning it but I have lots of anecdotal evidence that they remember what we talk about because it is tied together in their minds with vital skills that they love to use.
Integrating Play and Academic Content: Peter Gray and Suzanne Axelsson are two of my favorite educators. Both think, talk, and write extensively about the role of play in learning. I recommend both of them on my Substack. Both talk about the importance of free play for children and the learning that comes from such play. They also talk about a continuum from free play to playful information sharing. I leave lots of room for free play for kids of all ages during the activities I host. I also use games like Nature Tag - Tree Version, Nature Tag - Make Me One With Everything Version, Transformative Nature Treasure Hunts, The Interaction Game, The Build a System Game, The Hide Along the Trail Game, and the Coyotes Stalk a Deer Game to help kids learn academic content. Also, when I introduce new materials like a bow-drill set or ball-and-stick molecular modeling pieces, I give kids time to play with new materials before I give specific instructions. Maybe the most consequential way play enters our learning environment is our total disregard for what may be the worst truism to emerge from the US public school system: don’t smile until January. My relationships with kids, while always respectful, kind, and caring and always including some safety patrolling and lifeguarding, are also almost always playful.
Teacher-centered (sage on stage), Student-Centered, or Relationship and Earth-Centered Education: I talk a lot about the fact that when I’m with children, I don’t feel like what we do is child-centered, teacher-centered, or even some percentage of one and some percentage of the other. We sometimes describe what we do as relationship-centered or as sharing control. My friend Suzanne Axelsson, an educational thinker and writer, recently posted a stimulating blog post, The World at the Centre. In this post, she talks about shifting the focus of education from the child-centered/curriculum-centered dichotomy to a world-centered approach. Since I read her post, I’ve been playing around with the phrase Earth-centered education. I love the sound of this! One of the myths we spend a lot of time debunking with children is the idea of human exceptionalism. Child-centered and teacher-centered both put humans front and center. I am eager to play around more with the phrase Earth-centered education.
Wrap Up
This post began with a verse from the Haudenosaunee Thanksgiving Address. That verse points out that the Sun gives light to the Earth and is also the source of all the fires of life. We then suggested a sequence of activities and experiences that took us through light energy turning into heat energy, plants using the energy of the Sun to turn our outbreaths into sugars which store the Sun’s energy, using fire to experience releasing the plants stored up energy from the Sun as light and heat energy, and how the fires of life burning at low temperatures inside the mitochondria of all eukaryotic organisms also use oxygen to burn sugars releasing the stored energy of the Sun. We hope that these experiences and the science and educational ideas we discussed leave you with the idea that understanding energy is requisite to understanding both the geophysical and anthropogenic contributions to global climate change and the importance of learning traditional ecological knowledge and modern science side-by-side.






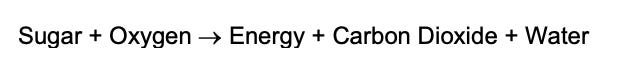
Just FYI, after listening to Nat Geo explorer Lee Berger speak at a book event, I no longer believe that building and circling around fires is uniquely human! If you’re interested in learning about their recent discoveries look up Homo Naledi — even though homo is in their name, their bodies were more like animals than humans, yet they made fires and had funerals way before we have knowledge of human ancestors ever doing the same. There’s also a documentary about them.